Describe Rutherford's Gold Foil Experiment
Atoms and Elements Related Topics. In Bohrs model the orbits of the electrons were explained by quantum mechanics.
Size Of The Nucleus Rutherford Gold Foil Experiment
Charges and mass spread.
. First week only 499. Rutherford and the nucleus. He realized this because most of the alpha particles passed straight.
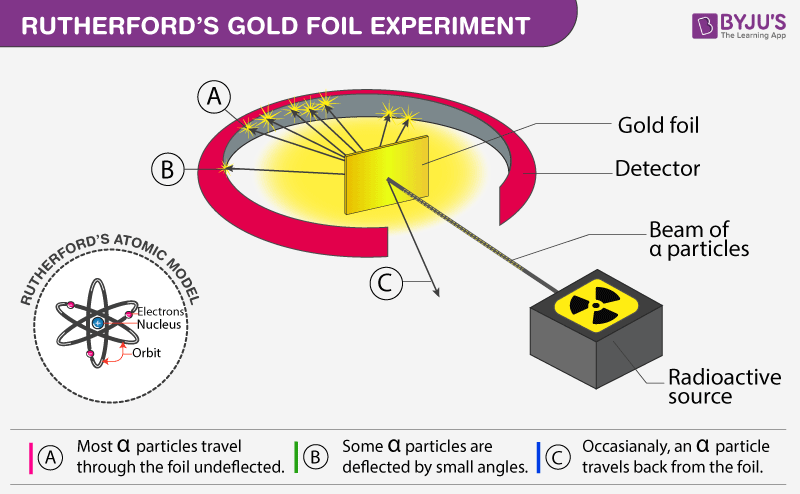
Describe Rutherfords gold foil experiment and the results of that experiment. How did these results refute the plum pudding model of the atom. Rutherford passed beams of alpha particles through a thin gold foil and noted how the alpha particles scattered from the foil.
They bombarded very thin sheets of gold foil with fast moving alpha particles. Thomsons plum pudding model viewed the atom as a massive blob of positive charge dotted with negative charges. In the experiment Rutherford passes very high streams of alpha-particles from a radioactive source ie.
In his experiment he bombarded a thin gold foil with a beam of alpha particles particles carrying two units of positive charge and four units of mass and observed their behavior with the help of a screen coated. Observations of Rutherfords alpha ray scattering experiment. His two students Hans Geiger and Ernest Marsden directed a beam of.
His two students Hans Geiger and Ernest Marsden directed a beam of alpha particles at a. To make their observations Rutherford and his students. Figure PageIndex2 a The experimental setup for Rutherfords gold foil experiment.
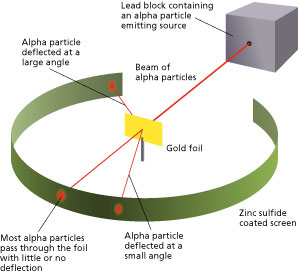
So think of the model as a spherical Christmas cake. In 1905 Ernest Rutherford did an experiment to test the plum pudding model. Most of the α-particles passed straight through the gold foil without any deviation.
Solution for Describe Rutherfords gold foil experiment. Alpha particles a type of natural radioactive particle are positively charged particles with a. B According to the plum pudding model top all of the alpha particles should have passed through the gold.
Khan Academy is a 501c3 nonprofit organization. Rutherford in his experiment directed high energy streams of α-particles from a radioactive source at a thin sheet 100 nm thickness of gold. About Rutherfords Gold Foil Experiment History.
A plum pudding was a Christmas cake studded with raisins plums. Before the experiment the best model of the atom was known as the Thomson or plum pudding model. Rutherford Gold Foil Experiment.
In 1911 Rutherford and coworkers Hans Geiger and Ernest Marsden initiated a series of groundbreaking experiments that would completely change the accepted model of the atom. Niels Bohr built upon Rutherfords model to make his own. A radioactive element that emitted alpha particles was directed toward a thin sheet of gold foil that was surrounded by a screen which would allow detection of the deflected particles.
The essential idea of Rutherfords theory is to consider the -particle as a charged mass traveling. Rutherfords gold foil experiment Our mission is to provide a free world-class education to anyone anywhere. In this model the atom was believed to consist of a positive material pudding with negative plums distributed throughout.
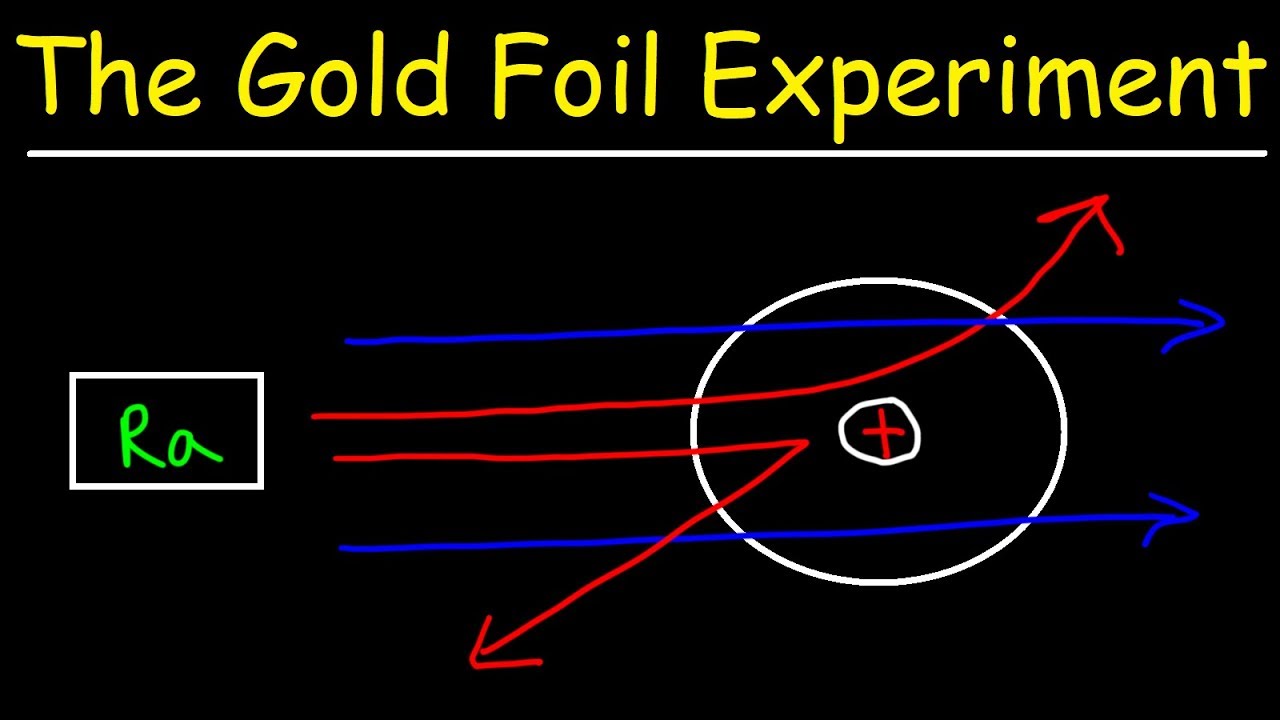
When Rutherford shot α particles through gold foil he found that most of the particles went through. Alpha-particle emitter at a thin sheet of100 nm thickness of gold. He conduct an experiment by bombarding alpha particles into a thin sheet of gold and then notices their interaction with the gold foil and trajectory or path followed by these particles.
This image shows the cage with alpha source and gold scattering foil prepared for a. Some of the α-particles were deflected by the foil by some angles. In 1905 Ernest Rutherford did an experiment to test the plum pudding model.
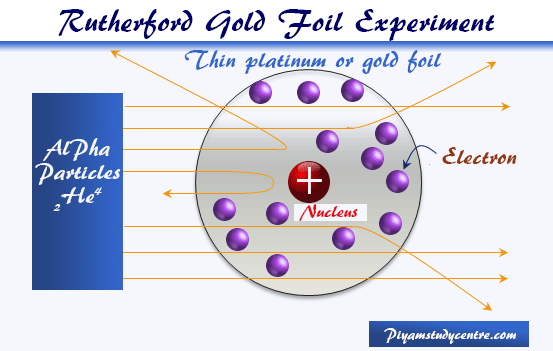
Rutherford directed beams of alpha particles which are the nuclei of helium. The gold-foil experiment showed that the atom consists of a small massive positively charged nucleus with the negatively charged electrons being at a great distance from the centre. Thompson the scientist who discovered the electron.
Before Rutherfords experiment the best model of the atom that was known to us was the Thomson or plum pudding model. Rutherfords conducted an experiment by bombarding a thin sheet of gold with α-particles and then studied the trajectory of these particles after their interaction with the gold foil. Later Rutherfords alpha-particle scattering.
The evidence for the existence of the nucleus has been provided by Rutherfords Experiment which he carried out in 1911. The atom was believed to consist of a positive material pudding with negative plums distributed throughout. Alpha beam and this can be covered with gold foil to fully simulate the energy loss in the gold foil scattering experiment.
Weve got the study and writing resources you need for your assignments. Rutherfords gold foil experiment Rutherfords alpha particle scattering experiment refers. Rutherfords alpha particle scattering experiment changed the way we think of atoms.
Rutherfords gold foil experiment showed that atoms are mostly empty space with the positive charge concentrated in a nucleus. The Rutherford gold foil experiment worked by firing positively charged alpha particles through gold foil and observing where they ended up. The Gold Foil Experiment.
The gold foil experiment was conducted under the supervision of Rutherford at the University of Manchester in. Rutherford Gold Foil Experiment. The GeigerMarsden experiments also called the Rutherford gold foil experiment were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated.
This model was developed in 1904 by JJ. Start your trial now.

Rutherford S Atomic Model Physics8atlaurel

Gold Foil Experiment Overview Importance Expii
Experimental Evidence For The Structure Of The Atom
Rutherford S Gold Foil Experiment Quick And Simple Youtube

Atom Rutherford S Nuclear Model Britannica
What Was Rutherford S Gold Foil Experiment Quora
Rutherford Model Experiment Observation Limitation
Lord Rutherford The History Of The Atom

Rutherford S Atomic Model Chemistry For Non Majors

Rutherford S Gold Foil Experiment Chemistrygod

Ernest Rutherford S Gold Foil Experiment Physics Lab Video Lesson Transcript Study Com
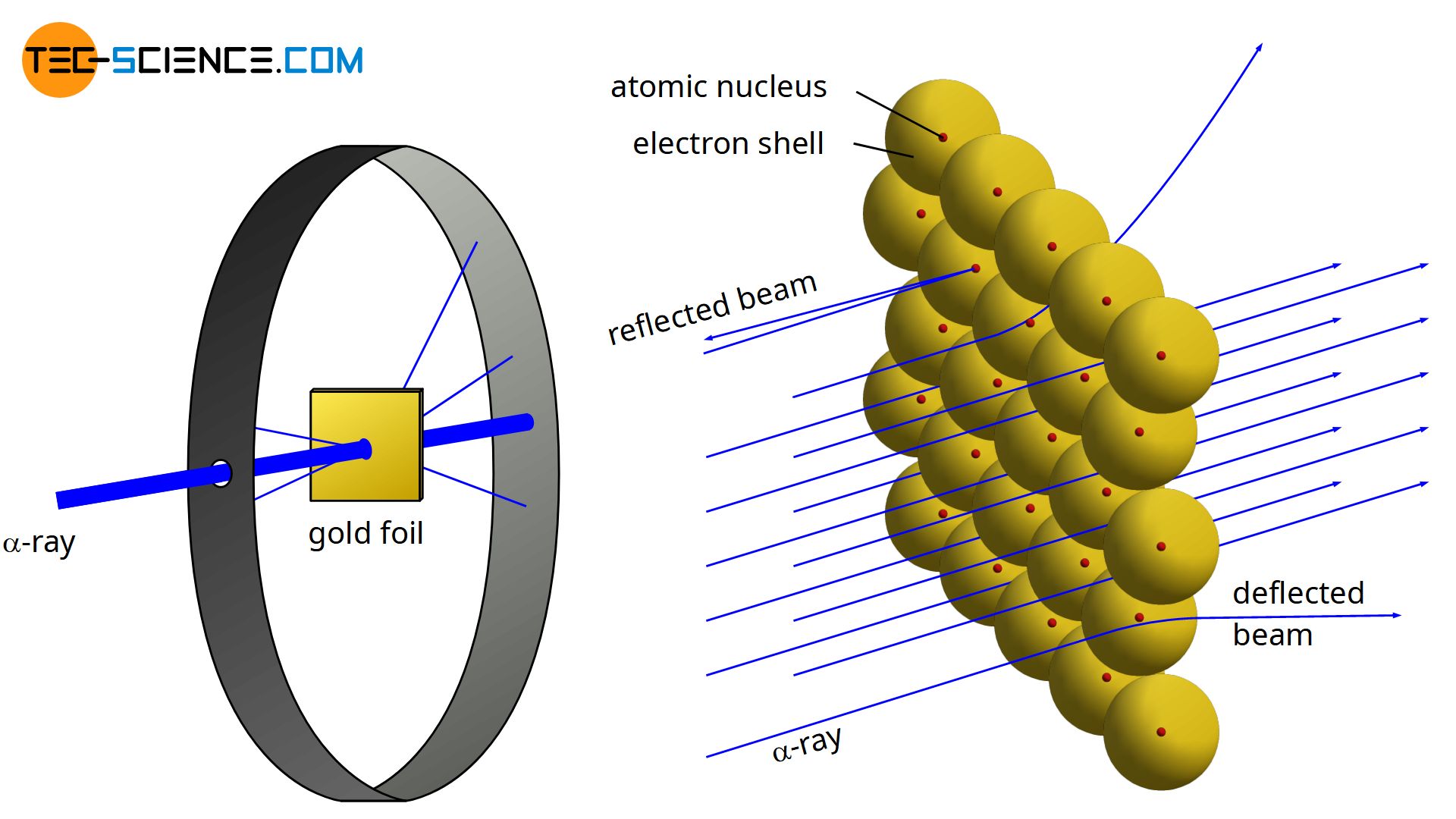
Rutherford S Atomic Model Tec Science
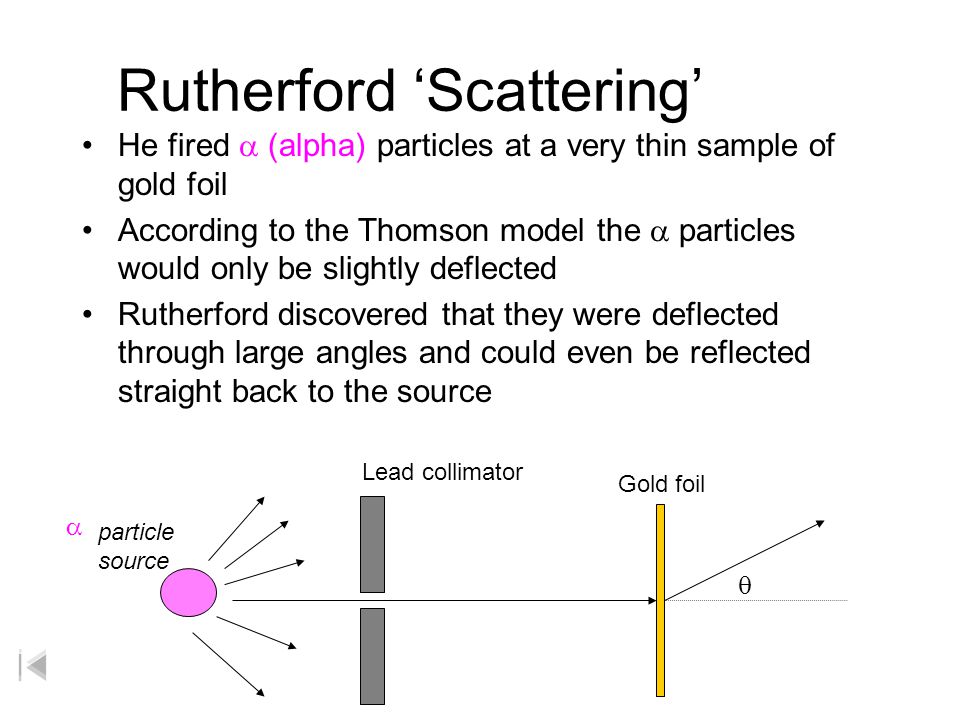
Rutherford S Gold Foil Experiment Ppt Video Online Download

Ernest Rutherford S Gold Foil Experiment Atomic Theory Modern Physics Ernest Rutherford

Rutherford S Atomic Model Gold Foil Experiment Results Applications
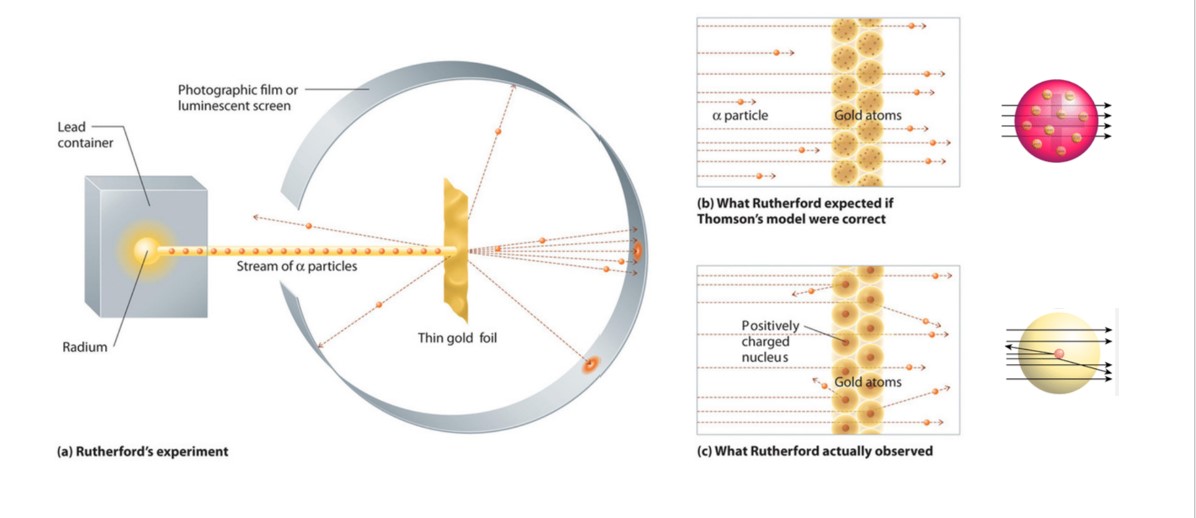
3 4 Rutherford S Experiment The Nuclear Model Of The Atom Chemistry Libretexts

Rutherford S Gold Foil Experiment
What Happens When Alpha Particles Were Directed At A Piece Of Gold Foil Quora

Rutherford S Gold Foil Experiment Setup Analysis And Conclusion
Comments
Post a Comment